TILDREN®
(tiludronate disodium)
Bisphosphonate drug for intravenous infusion. For use in horses only.
Description:
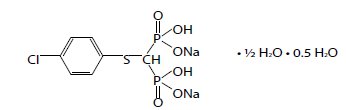
TILDREN® contains the sodium salt of tiludronic acid, which is a bisphosphonate characterized by a (4-chlorophenylthio) group on the carbon atom of the basic P-C-P structure common to all bisphosphonates. Its United States Adopted Name (USAN) is tiludronate disodium. Tiludronate disodium is the hydrated hemihydrate form of the disodium salt of tiludronic acid. Its chemical name is [[(4-Chlorophenyl) thio]methylene] bis[phosphonic acid], disodium salt, and its structural formula is as follows:
TILDREN is a sterile powder. Each vial of TILDREN contains 500 mg of tiludronic acid (as tiludronate disodium) and 250 mg mannitol USP (excipient).
Indications:
TILDREN is indicated for the control of clinical signs associated with navicular syndrome in horses.
Dosage and Administration:
A single dose of TILDREN should be administered as an intravenous infusion at a dose of 1 mg/kg (0.45 mg/lb). The infusion should be administered slowly and evenly over 90 minutes to minimize the risk of adverse reactions.
Maximum effect may not occur until 2 months post-treatment.
Directions for Administration:
Do not reconstitute or mix TILDREN with calcium containing solutions or other solutions containing divalent cations such as Lactated Ringers as it may form complexes with these ions.
STEP 1: Preparation of the reconstituted solution (20 mg/mL). TILDREN should be reconstituted using strict aseptic technique. Remove 25 mL of solution from a 1 liter bag of sterile 0.9% Sodium Chloride Injection, USP and add it to one vial of TILDREN. Shake gently until the powder is completely dissolved. This reconstituted solution contains 20 mg of tiludronate disodium per mL. After reconstitution further dilution is required before administration.
STEP 2: Preparation of the solution for infusion. Using strict aseptic technique, withdraw the appropriate volume of the reconstituted solution based on the horse’s body weight (see Table 1 below). Inject that volume back into the 1 liter bag of sterile 0.9% Sodium Chloride Injection, USP. Horses greater than 1,210 lbs. will require a second vial of reconstituted TILDREN solution. Invert the infusion bag to mix the solution before infusion. Label the infusion bag to ensure proper use.
After preparation, the infusion should be administered either within 2 hours of preparation, or it can be stored for up to 24 hours under refrigeration at 36-46° F (2-8° C) and protected from light.
STEP 3: Administer for infusion through a suitable intravenous catheter inserted into a jugular vein and connected to the infusion bag using sterile disposable infusion tubing.
Table 1: Dosing Table
Weight Range (lbs) |
Volume of Reconstituted TILDREN Solution (20 mg/mL) to be added to the infusion bag |
550-770 |
15 mL |
771-990 |
20 mL |
991-1210 |
25 mL* |
1211-1430 |
30 mL (25 mL* + 5 mL from additional vial) |
1431-1650 |
35 mL (25 mL* + 10 mL from additional vial) |
1651-1875 |
40 mL (25 mL* + 15 mL from additional vial) |
*1 vial = 25 mL
Contraindications:
Do not use in horses with known hypersensitivity to tiludronate disodium or to mannitol.
Do not use in horses with impaired renal function or with a history of renal disease.
Bisphosphonates are excreted by the kidney; therefore, conditions causing renal impairment may increase plasma bisphosphonate concentrations resulting in an increased risk for adverse reactions.
Warnings:
Do not use in horses intended for human consumption.
NSAIDs should not be used concurrently with TILDREN. Concurrent use of NSAIDs with TILDREN may increase the risk of renal toxicity and acute renal failure. Acute renal failure has been reported in horses concurrently administered NSAIDs and TILDREN within a 48 hour period. Additionally, horses concurrently administered TILDREN and NSAIDs in field studies demonstrated a statistically significant increase in serum blood urea nitrogen (BUN) and creatinine concentrations. These elevations were not always associated with clinical signs of renal dysfunction. Therefore, appropriate wash-out periods should be observed between NSAID and TILDREN administration, and BUN and creatinine should be monitored. If treatment for discomfort is required after TILDREN administration, a non-NSAID treatment should be used.
HUMAN WARNINGS
Not for human use. Keep this and all drugs out of the reach of children. Consult a physician in case of accidental human exposure.
Precautions:
Approximately 30-45% of horses administered TILDREN will demonstrate transient signs consistent with abdominal pain (colic). Clinical signs usually occur shortly after TILDREN administration and may be associated with alterations in intestinal motility. Horses should be observed closely for 4 hours post-infusion for the development of clinical signs consistent with colic or other adverse reactions. Colic signs can last approximately 90 minutes and may be intermittent in nature. Hand-walking the horse may improve or resolve the colic signs in many cases. If a horse requires medical therapy, non-NSAID treatments should be administered due to the risk for renal toxicity. Avoid NSAID use. (See Warnings.)
TILDREN should be administered slowly and evenly over 90 minutes to minimize the risk of adverse reactions. Field studies demonstrated a higher incidence of adverse reactions when the infusion was administered over shorter periods of time.
Horses should be well hydrated prior to administration of TILDREN due to the potential nephrotoxic effects of TILDREN.
Concurrent administration of other potentially nephrotoxic drugs should be approached with caution, and if administered, renal function should be monitored.
TILDREN affects plasma concentrations of certain minerals and electrolytes, including calcium, magnesium and potassium, immediately post-treatment, with effects lasting up to several hours. Caution should be used when administering TILDREN to horses with conditions affecting mineral or electrolyte homeostasis (e.g. hyperkalemic periodic paralysis (HYPP), hypocalcemia, etc.) and conditions which may be exacerbated by hypocalcemia (e.g. cardiac disease).
TILDREN should be used with caution in horses receiving concurrent administration of other drugs that may reduce serum calcium (such as tetracyclines) or whose toxicity may exacerbate a reduction in serum calcium (such as aminoglycosides).
Horses with HYPP (heterozygous or homozygous) may be at an increased risk for adverse reactions, including colic signs, hyperkalemic episodes, and death. In one field study, one horse that was an unknown carrier (heterozygous) for HYPP died after receiving TILDREN. In a study evaluating the safety of TILDREN in horses with HYPP, an increased percentage of horses showed colic signs following the second TILDREN infusion (See Animal Safety). Genetic testing for HYPP in horses with possible or known Impressive bloodlines should be considered prior to the administration of TILDREN.
The safe use of TILDREN has not been evaluated in horses less than 4 years of age. The effect of bisphosphonates on the skeleton of growing horses has not been studied; however, bisphosphonates inhibit osteoclast activity which impacts bone turnover and may affect bone growth.
Bisphosphonates should not be used in pregnant or lactating mares, or mares intended for breeding. The safe use of TILDREN has not been evaluated in pregnant or lactating mares, or in breeding horses; however, bisphosphonates are incorporated into the bone matrix, from where they are gradually released over periods of months to years. The extent of bisphosphonate incorporation into adult bone, and hence, the amount available for release back into the systemic circulation, is directly related to the total dose and duration of bisphosphonate use. Bisphosphonates have been shown to cause fetal developmental
abnormalities in laboratory animals. The uptake of bisphosphonates into fetal bone may be greater than into maternal bone creating a possible risk for skeletal or other abnormalities in the fetus. Many drugs, including bisphosphonates, may be excreted in milk and may be absorbed by nursing animals.
Increased bone fragility has been observed in laboratory animals treated with bisphosphonates at high doses or for long periods of time. Bisphosphonates inhibit bone resorption and decrease bone turnover which may lead to an inability to repair microdamage within the bone. In humans, atypical femur fractures have been reported in patients on long term bisphosphonate therapy; however, a causal relationship has not been established.
Adverse Reactions:
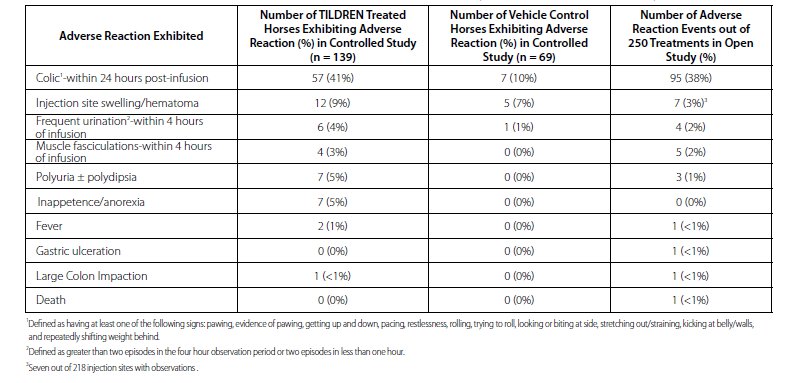
Three field studies were conducted evaluating the use of TILDREN in horses: a controlled field effectiveness study, a 6-month open use field study, and an infusion rate field safety study. In the controlled field effectiveness study, 139 horses were treated with a single intravenous infusion of TILDREN over 30-60 minutes and 69 horses were treated with vehicle control. Adverse reactions were most common during the infusion or within 4 hours following the end of the infusion (see Table 2). Fifty-seven TILDREN-treated horses (41%) and seven vehicle control horses (10%) exhibited at least one sign consistent with abdominal discomfort or colic (pawing, evidence of pawing, getting up and down, pacing, restlessness, rolling, trying to roll, looking at or biting at side, stretching out/straining, kicking at belly/walls, and shifting weight). Adverse reactions were also reported for horses in the controlled field effectiveness study that continued into a 6-month open use field study where 250 doses were administered to 97 horses. Incidence of adverse reactions was similar to the controlled field effectiveness study.
Table 2: Number of Adverse Reactions in the Controlled Field Effectiveness Study and in the 6-month Open Use Field Study
Adverse reactions occurring between 4 hours and 1 day post treatment in TILDREN-treated horses included: increased frequency of urination with or without increased drinking, reduced appetite, sore or stiff neck, fever, and uncomplicated colic.
Horses treated with an NSAID for post-TILDREN® infusion colic signs had statistically significant elevations in BUN and creatinine from baseline values. Additionally, 6/19 (32%) horses in the controlled field effectiveness study and 9/37 (24%) horses in the 6-month open use field study treated with an NSAID had elevations in creatinine above the normal reference range. These values returned to normal by the next evaluation, and were not associated with clinical signs of renal dysfunction.
Creatinine values in 3/139 (2%) TILDREN-treated horses were elevated above the reference range at intermittent time points during the controlled field effectiveness study. A similar percentage of horses in the 6-month open use field study (in which all horses received TILDREN) also had creatinine elevations. These elevations were transient and not associated with clinical signs of renal dysfunction.
During the 6-month open use field study, one horse with an unknown HYPP status had a severe episode of hyperkalemia (9.2 mEq/L) following TILDREN administration that resulted in death. Hyperkalemic periodic paralysis (HYPP) was confirmed by post-mortem genetic testing. Clinical signs associated with the hyperkalemic episode were vague, colic-like signs, muscle fasciculations, sweating, increased heart rate and respiratory rate, and ataxia. Presumptive cause of death was cardiac arrest due to severe hyperkalemia.
An infusion rate field safety study was conducted to evaluate the effect of slower infusion rate on incidence and severity of clinical signs of colic. In this study, 137 horses were administered a single dose of TILDREN over 90 (n = 69) or 120 (n = 68) minutes. Horses were observed continuously during the infusion and for at least 4 hours post treatment. Sixty (44%) horses exhibited clinical signs consistent with colic. There was no significant difference in the incidence or severity of colic between infusion groups. See Table 3 for a list of Adverse Reactions from the infusion rate field safety study.
Table 3. Adverse Reactions in Continuously Observed Horses Administered TILDREN over 90 or 120 minute infusion time (n = 137)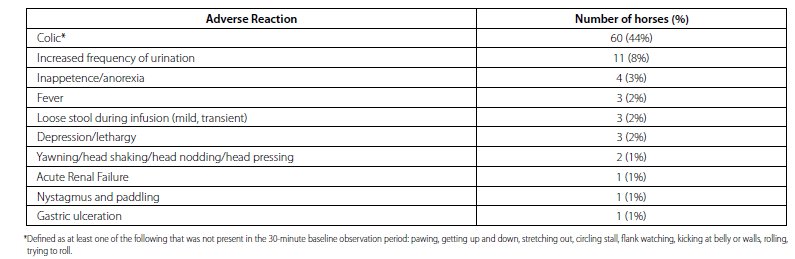
Colic signs were similar to those seen in previous studies. Pawing was the most common sign observed, followed by lying down. Clinical signs of colic tended to occur near the end of the infusion, or shortly after the end of the infusion. The average duration of colic signs was approximately 90 minutes and signs were often intermittent in nature. Forty-three (72%) horses with colic resolved with non-medical intervention, including hand-walking. Seventeen (28%) horses required at least one dose of a drug treatment for colic signs. Three of these horses required multiple drug treatments for resolution of signs. Horses that required treatment for discomfort were mostly treated with non-NSAIDs, including n-butylscopolammonium bromide and alpha-2 agonists, with or without an opioid. After multiple doses of non-NSAID drug treatments (including n-butylscopolammonium bromide, xylazine, and detomidine), one horse required treatment with an NSAID to resolve clinical signs of colic.
One horse that was administered an NSAID for fever several hours after the completion of TILDREN infusion was diagnosed with acute renal failure. Fluid therapy and diuresis were administered and the horse recovered. Azotemia resolved and BUN/creatinine returned to within the normal reference range 6 days following TILDREN administration.
Foreign Market Experience: Voluntary post-approval reporting of adverse reactions with the intravenous use of tiludronate disodium includes the following: polyuria, polydipsia, acute renal failure, chronic renal failure, anaphylaxis or hypersensitivity (including urticaria), enterotoxemia, muscle fasciculations, and fever. Death has been reported as the outcome of an adverse reaction listed above. Reports of acute renal failure most frequently included a history of co-administration of an NSAID within a short time frame of tiludronate disodium administration. To report suspected adverse events, for technical assistance or to obtain a copy of the MSDS, contact Ceva at 1-800-999-0297.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS (1-888-332-8387) or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Information for Owners: Prior to TILDREN (tiludronate disodium) administration, owners should be advised of the potential for adverse reactions in the hours or days following treatment. Adverse reactions within 4 hours post dosing may include signs of colic (manifested as pawing, stretching, getting up and down, sweating, rolling, looking at flanks, kicking at belly, frequent gas, and pacing), and less commonly yawning, licking lips, grinding teeth, muscle tremors, loose stool, decreased feed intake, and depression. Owners should allow the horse to lie down in a comfortable unrestricted area as recumbency can be experienced post infusion. Owners should watch their horse for increased water intake, increased urination, fever, and anorexia in the few days following TILDREN administration. Owners should be instructed to contact their veterinarian immediately if any adverse reactions are observed. Owners should be advised to consult with their veterinarian prior to the administration of an NSAID following TILDREN administration.
Clinical Pharmacology:
Pharmacokinetics: The plasma disposition of TILDREN (tiludronate disodium) was evaluated in horses following single intravenous administration as a 30-minute infusion at a dose of 1 mg/kg in order to calculate the major pharmacokinetic parameter values (Table 4).
Table 4. Pharmacokinetic parameter values for tiludronate disodium following IV administration as a 30-minute infusion of 1 mg/kg body weight to horses (n = 12)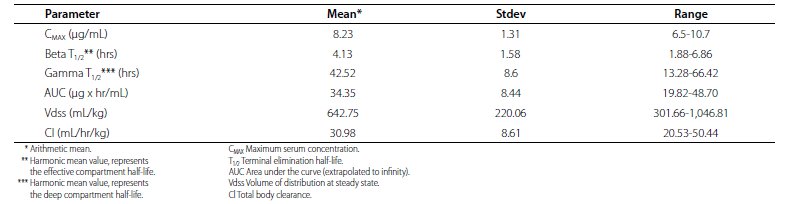
In the range of 0.33 to 3X of the recommended dose, Cmax is proportional to dose while the increase in AUC is slightly greater than dose-proportional. Clearance remains essentially constant. However, terminal elimination half life and volume of distribution increase with dose, indicating complex distribution kinetics. In the horse, plasma clearances estimated following each of 3 administrations of 1 mg/kg tiludronate disodium administered 14 days apart did not significantly differ. The mean accumulation factor based upon a comparison of the plasma AUC (measured from time zero to the last quantifiable concentration (AUC0-last)) for 2nd and 3rd infusions of 1 mg/kg tiludronate disodium 14 days apart was 1.1, indicating no significant plasma accumulation over the 3 infusions. A study performed at 0.1 mg/kg (0.1X dose) administered IV determined that the tiludronate disodium equine plasma protein binding is 81%. Following intravenous administration of 0.1 mg/kg (0.1X dose) tiludronate disodium for 10 consecutive days, there remained quantifiable bone concentrations of tiludronate disodium at approximately 6 months. Tiludronate disodium undergoes minimal metabolism and urine is the main route of excretion in the horse. Tiludronate disodium undergoes minimal metabolism and urine is the main route of excretion in the horse.
Pharmacodynamics:In vitro studies indicate that tiludronate disodium acts primarily on bone through a mechanism that involves inhibition of osteoclastic activity with a probable reduction in the enzymatic and transport processes that lead to resorption of the mineralized matrix. Tiludronate disodium exerts its inhibitory effect on bone resorption by blocking some of the osteoclast metabolic pathways: production of non-hydrolyzable, cytotoxic, ATP-analogue metabolites1, inhibition of the organization of the cytoskeleton required for the activation of the osteoclast2, and inhibition of the osteoclastic proton pumps3. Although a PK/PD model of the relationship between plasma and bone concentrations has not been developed, a study by Delguste et al.4 to compare tiludronate disodium bone concentrations using two different sampling techniques suggests that tiludronate disodium can persist in bone up to 1 year post-treatment.
Effectiveness: In a multicenter, placebo-controlled, masked, 2-month field study, 208 horses were randomly allocated to receive either a single dose of TILDREN (tiludronate disodium) plus corrective
shoeing or vehicle control plus corrective shoeing. Navicular syndrome was diagnosed based on a complete lameness examination, flexion tests, palmar digital nerve block, radiographs and MRI examination of both front feet. Horses enrolled in this study had navicular bone edema on MRI, and only mild soft tissue lesions. The majority of horses enrolled in the field study had a history of lameness of less than 6 months duration.
Of 835 screened horses, 208 (25%) met all the inclusion criteria and received treatment (139 TILDREN and 69 control). 181 horses (119 TILDREN and 62 control) were included in the effectiveness statistical analysis. A horse was considered a treatment success if the lameness in the primarily affected limb improved by at least 1 lameness grade, and there was no worsening of lameness grade in the other forelimb at 2 months post-treatment when compared to the pre-treatment assessment. Based on the statistical analysis, the estimated least squares mean success rates were 63.87% and 48.39% for the TILDREN and vehicle control groups, respectively. The difference in success rates was significant at p = 0.0479.
Animal Safety: In a target animal safety study, TILDREN was administered 3 times at monthly intervals to groups of 6 healthy horses at doses of 0 (control), 1, 3, and 5 times the recommended dose). A dose dependent increase in the incidence of clinical signs of colic was seen following TILDREN infusion at the rate of 2-4 mg/kg/hr. Decreased serum ionized calcium was seen immediately following treatment infusion, and extending up to 72 hours post-treatment in all treatment groups; however, the decreases were more consistent and more pronounced in the 3X and 5X treatment groups. Increased serum inorganic phosphate was seen 72 hours post-infusion in the 5X and occasionally the 3X groups. Mild increases in serum BUN and creatinine were noted in all treatment groups.
In a study to evaluate safety in horses carrying the gene for HYPP, 12 horses heterozygous (N/H) for the genetic trait were administered 2 doses of TILDREN or saline 30 days apart at a dose of 1 mg/kg (1X) infused over 90 minutes. Horses were observed continuously during the infusion and for 4 hours post infusion. None of the horses administered TILDREN had confirmed hyperkalemia during the immediate post-infusion period. One control horse and two TILDREN horses exhibited signs consistent with possible hyperkalemia (mild muscle fasciculations) but had normal serum potassium and signs resolved with or without administration of oral corn syrup. Five horses (71%) exhibited clinical signs of colic following the second infusion of TILDREN. Clinical signs were consistent with what has been observed in previous studies and all cases resolved with non-medical intervention, including hand-walking.
Storage Information:
Sterile powder (not reconstituted): Store at controlled room temperature 68-77° F (20-25° C). After preparation, the infusion should be administered either within 2 hours of preparation, or it can be stored for up to 24 hours under refrigeration at 36-46° F (2-8° C) and protected from light.
References
1. Russell et al., The Pharmacology of Bisphosphonates and New Insights Into Their Mechanisms of Action. 1999. J of Bone Min Res. 14(2); 53-65.
2. Murakami et al., Tiludronate Inhibits Protein Tyrosine Phosphatase Activity in Osteoclasts. 1997. Bone 29(5); 399-404.
3. David et al., The Bisphosphonate Tiludronate is a Potent Inhibitor of the Osteoclast Vacuolar H+-ATPase. 1996. J of Bone Min Res.11(10); 1498-1507.
4. Delguste et al., Assessment of a bone biopsy technique for measuring tiludronate in horses: A preliminary study. 2011. Canadian J of Vet. Res. 75:128-133.
How Supplied:
TILDREN is supplied in a 30 mL glass vial as a white, sterile lyophilized powder containing 500 mg tiludronic acid (as tiludronate disodium) packaged in a folding carton. For technical assistance or to report suspected adverse reactions, call 1-800-999-0297.
Marketed by Ceva Animal Health, LLC Lenexa, KS 66215
Made in Canada
Patent information: U.S. patent 6,057,306
NDC 13744-800-50
NADA #141-420, Approved by FDA
Tildren® is a registered trademark of Ceva Santé Animale, France
C518US 08-c1-v2
Rev. 04/2014
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Tildren ® (tiludronate disodium)
Bisphosphonate drug for intravenous infusion.
For use in horses only.
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Net contents: 500 mg
NADA 141-420, Approved by FDA
Tildren® is a registered trademark of Ceva Sante Animale, France
C518US 03-c1-v2
Carton Label