For Topical Application in Dogs Only
Opioid Analgesic
NOT FOR INJECTION
50 mg/mL (500 mg/10 mL)
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
WARNING:
Abuse Potential:
RECUVYRA contains fentanyl, a high concentration μ-opioid receptor agonist (50 mg/mL) and is a Class II controlled substance with high potential for abuse similar to hydromorphone, methadone, morphine, oxycodone, and oxymorphone. Class II opioid substances have the highest potential for human abuse and criminal diversion. The high concentration of RECUVYRA may be a particular target for human abuse. Fentanyl has additive CNS depressant effects when used with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Fatal fentanyl overdoses in humans are due to respiratory depression.
The risk of abuse by humans should be considered when storing, administering, and disposing of RECUVYRA. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (suicidal depression).
Risk Minimization and Action Plan:
This product is distributed under a Risk Minimization Action Plan (RiskMAP) and its use is limited to certified veterinarians.
Human Safety:
SECONDARY EXPOSURE TO FENTANYL IN CHILDREN AND ADULTS:
Strict adherence to the requirements of the RiskMAP and the INSTRUCTIONS FOR USE provided in this product insert is imperative in order to reduce the potential of secondary exposure to fentanyl from RECUVYRA treated skin.
- The dog should be isolated from children for 72 hours (3 days) from the time of RECUVYRA application to the dog.
- If a child comes in direct contact with the dog within 72 hours (3 days) from the time of RECUVYRA application to the dog, the exposed part of the child's body should not contact the child's mouth or eyes, and the area should be washed with soap and water.
- If a child's tongue comes in contact with the dog, or another part of the child's body comes in contact with the dog and is then placed in the mouth, it is possible for fentanyl to enter the bloodstream; this is a medical emergency and the child should be seen immediately by a physician.
- Adults should avoid contact with the application site for 72 hours (3 days) following the application of RECUVYRA to the dog. Within this period, any part of the body that directly contacts the application site should be washed with soap and not inserted into the mouth.
- The antidote for human exposure to RECUVYRA transdermal solution is an opioid reversal agent such as naltrexone or naloxone.
Animal Safety:
Individual dogs especially sensitive to the effects of fentanyl may develop gastrointestinal stasis with an increased risk of bacterial overgrowth, accompanied by pronounced sedation, and decreased intake of food and water. Dehydration and elevations in hematocrit, albumin, and fibrinogen may occur. Feces, if present, could be soft and contain blood. In the event of severe gastrointestinal stasis, an
opioid reversal agent should be administered to the dog (see PRECAUTIONS) with intravenous fluids and other supportive measures.
RECUVYRA is contraindicated for dogs with diseased or injured dorsal scapular epidermis, dogs expected to have mild or absent perioperative pain, dogs with paralytic ileus, and dogs with known hypersensitivity to fentanyl.
See Contraindications, Warnings: Human and Animal Safety, and Precautions for detailed information.
RISK MINIMIZATION & ACTION PLAN
The RECUVYRA Risk Minimization Action Plan (RiskMAP) provides educational materials to the veterinarian, veterinary staff, and the dog owner explaining the risks and proper use of RECUVYRA. Once it is documented that the dog owner has read and understood the materials by signing the client information sheet, the drug can be applied to the patient. Veterinarians are expected to report all serious adverse events that occur in animals or humans to the manufacturer (see WARNINGS).
RECUVYRA is only for use in dogs, and available only through a restricted distribution program limited to certified distributors that are trained to distribute RECUVYRA under the conditions of the RiskMAP. RECUVYRA can only be received and
administered by veterinarians through the restricted distribution program because of the potential for human abuse and safety risks associated with its use in dogs.
DESCRIPTION
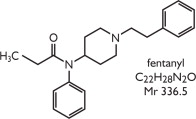
RECUVYRA is a volatile liquid transdermal solution intended for topical application that provides continuous, systemic delivery of a potent analgesic opioid. RECUVYRA is a clear, colorless to light yellow solution that contains 5% w/v (50 mg/mL) fentanyl as the active pharmaceutical ingredient in alcohol and octyl salicylate. Fentanyl is a μ (mu) opioid receptor agonist. Once applied to the skin, RECUVYRA is a rapid drying formulation resulting in fast dermal absorption and sequestration of fentanyl in the stratum corneum.
INDICATIONS
RECUVYRA is indicated for the control of postoperative pain associated with surgical procedures in dogs.
DOSAGE AND ADMINISTRATION
NOT FOR INJECTION
The dosage of RECUVYRA is 1.2 mg/lb (2.7 mg/kg) applied topically to the dorsal scapular area 2 to 4 hours prior to surgery. A single application provides analgesia for 4 days. Use the provided syringe and applicator tips. Apply RECUVYRA only once. Accumulation of fentanyl following repeated administration could result in severe adverse reactions, including death (see ANIMAL SAFETY).
Do not dispense RECUVYRA for administration at home by the pet owner.
RECUVYRA should only be handled and administered to dogs by hospital staff specially trained in its use. To prevent human adverse reactions or abuse, at least 2 trained administrators should be present during administration of RECUVYRA. Wear impermeable latex or nitrile gloves, protective glasses and a laboratory coat to prevent direct contact with human skin, eyes or mucosa when handling and/or applying the solution. Do not administer RECUVYRA outside of the hospital setting. Use only the syringes provided and do not store RECUVYRA in the syringe. Use of syringes incompatible with RECUVYRA will result in inaccurate dosing or human exposure (see Instructions for Use for details regarding drug application and restraint during drying time).
* RECUVYRA cannot be accurately dosed in dogs less than 6 pounds of body weight. The average dose in each weight band is approximately 2.7 mg/kg, except for the lowest weights, that average slightly higher. | ||
| POUNDS OF BODY WEIGHT | DOSE (mL) |
KILOGRAMS OF BODY WEIGHT |
| 6 to 9.3* | 0.2 | 2.7 to 4.2 |
| 9.4 to 13.4 | 0.3 | 4.3 to 6.1 |
| 13.5 to 17.6 | 0.4 | 6.2 to 8 |
| 17.7 to 21.8 | 0.5 | 8.1 to 9.9 |
| 21.9 to 25.9 | 0.6 | 10 to 11.7 |
| 26 to 30.1 | 0.7 | 11.8 to 13.6 |
| 30.2 to 34.3 | 0.8 | 13.7 to 15.5 |
| 34.4 to 38.4 | 0.9 | 15.6 to 17.4 |
| 38.5 to 42.6 | 1 | 17.5 to 19.3 |
| 42.7 to 46.8 | 1.1 | 19.4 to 21.2 |
| 46.9 to 50.9 | 1.2 | 21.3 to 23.1 |
| 51 to 55.1 | 1.3 | 23.2 to 25 |
| 55.2 to 59.3 | 1.4 | 25.1 to 26.9 |
| 59.4 to 63.4 | 1.5 | 27 to 28.8 |
| 63.5 to 67.6 | 1.6 | 28.9 to 30.6 |
| 67.7 to 71.8 | 1.7 | 30.7 to 32.5 |
| 71.9 to 75.9 | 1.8 | 32.6 to 34.4 |
| 76 to 80.1 | 1.9 | 34.5 to 36.3 |
| 80.2 to 84.3 | 2 | 36.4 to 38.2 |
| 84.4 to 88.4 | 2.1 | 38.3 to 40.1 |
| 88.5 to 92.6 | 2.2 | 40.2 to 42 |
| 92.7 to 96.8 | 2.3 | 42.1 to 43.9 |
| 96.9 to 100.9 | 2.4 | 44 to 45.8 |
| 101 to 105.1 | 2.5 | 45.9 to 47.7 |
| 105.2 to 109.3 | 2.6 | 47.8 to 49.6 |
| 109.4 to 113.4 | 2.7 | 49.7 to 51.4 |
| 113.5 to 117.6 | 2.8 | 51.5 to 53.3 |
| 117.7 to 121.8 | 2.9 | 53.4 to 55.2 |
| 121.9 to 125 | 3 | 55.3 to 57 |
Apply up to ½ mL onto the skin without moving the applicator tip. If the calculated volume is greater than ½ mL, reposition the applicator tip at least 1 inch from the initial site and apply up to ½ mL. Repeat the reposition and application steps until the entire calculated volume has been applied to the dog. Restrain the dog for a full 2 minutes and avoid contact with the application site for 5 minutes to allow complete drying of the solution (SEE INSTRUCTIONS FOR USE).
INSTRUCTIONS FOR USE
RECUVYRA should only be handled and administered to dogs in a clinic setting by hospital staff specially trained in its use. To prevent human adverse reactions or abuse, at least 2 trained administrators should be present during administration of RECUVYRA.
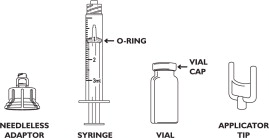
COMPONENTS:
STEP 1:
PREPARATION OF THE HANDLER
Wear impermeable latex or nitrile gloves, protective glasses and a laboratory coat to prevent direct contact with human skin, eyes or mucosa when handling and/or applying the solution.
STEP 2:
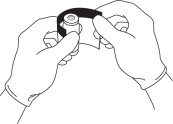
COMPLETELY REMOVE THE SHRINK WRAP COVER FROM THE VIAL
Grasp the edge of the shrink wrap at the top of the vial and pull to remove.
STEP 3:
REMOVE THE PLASTIC PROTECTIVE COVER FROM THE VIAL CAP
Grasping the vial, pry the cover off the vial cap by hand.
STEP 4:
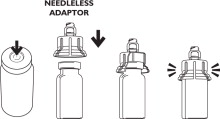
ATTACH THE NEEDLELESS ADAPTOR TO THE VIAL
Place the vial upright on a firm, stable surface during placement and application of the needleless adaptor. Center the needleless adaptor directly over the top of the vial. Applying gentle, even pressure, press the needleless adaptor onto the vial until it is seated. Once seated, do not remove the needleless adaptor.
On the vial where it states, DISCARD AFTER, write the date that is 30 days from the date that the needleless adaptor was applied. Store vial upright with the needleless adaptor attached.
STEP 5:
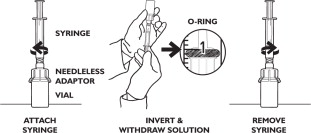
WITHDRAW DRUG FROM THE VIAL
To withdraw RECUVYRA from the vial, press the syringe tip into the center of the needleless adaptor and gently turn syringe approximately 1/4 turn clockwise until fully seated. Invert vial and pull back on syringe plunger until the appropriate volume is withdrawn. It may be necessary to evacuate air from the syringe back into the vial. To accurately withdraw the correct amount, align the top of the O-ring on the syringe plunger with the appropriate tick mark on the syringe barrel. To remove the syringe from the adaptor, turn the vial into the upright position, grasp the needleless adaptor, turn the syringe 1/4 turn counterclockwise and remove the syringe.
STEP 6:
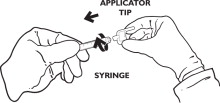
ATTACH APPLICATOR TIP TO SYRINGE
Attach the applicator tip to the syringe by turning the applicator tip 1/3 turn clockwise.
STEP 7:
SITE PREPARATION
It is not necessary to clip the hair over the application site. However, in dogs with thick haircoats, close clip the hair on the dorsal scapular area prior to application to ensure direct contact of RECUVYRA with the skin. The application site should be free of gross debris and the skin should be intact and free from disease or injury.
STEP 8:
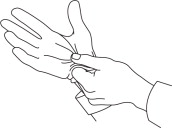
APPLY RECUVYRA TO THE DOG
Place applicator tip at an approximate 45º angle directly onto the skin in the dorsal scapular area. It is important that both tips make direct contact with the skin.
Apply up to 1/2 mL onto the skin without moving the applicator tip. If the calculated volume is greater than 1/2 mL, reposition the applicator tip at least 1 inch from the initial site and apply up to 1/2 mL. Repeat the reposition and application steps until the entire calculated volume has been applied to the dog.
Restrain the dog for a full 2 minutes and avoid contact with the application site for 5 minutes to allow complete drying of the solution. Dispose of the used syringe/applicator tip as a unit in an appropriate container. Disposal should comply with Drug Enforcement Agency (DEA) guidelines.
STORAGE AND DISCARD:
Once broached with the needleless adaptor, store the vial upright at controlled room temperature, 20 - 25ºC (68 - 77ºF), with the needleless adaptor attached.
RECUVYRA is a Class II opioid (see WARNINGS, Human Safety: Drug abuse, addiction and diversion of opioids). Store in a locked, substantially constructed cabinet according to DEA and local controlled substance guidelines.
Discard broached vials after 30 days. Any unused or expired vials must be destroyed by a DEA registered reverse distributor; for further information call 1-888-545-5973.
CONTRAINDICATIONS
Do not apply RECUVYRA to skin that is diseased or injured.
Do not administer RECUVYRA to anatomic areas other than the dorsal scapular area because absorption characteristics may be different.
Do not administer RECUVYRA to dogs where postoperative pain is expected to be mild or absent.
Do not administer RECUVYRA to dogs that have or are suspected of having paralytic ileus.
Do not administer RECUVYRA to dogs that are hypovolemic or debilitated.
Do not administer RECUVYRA to dogs with a known hypersensitivity to fentanyl.
Do not administer a second dose of RECUVYRA. Accumulation of fentanyl following repeated administration could result in severe adverse reactions, including death (see ANIMAL SAFETY).
Do not administer RECUVYRA to any other species; it is intended for use in dogs only. Safe and effective concentrations of fentanyl are dependent on appropriate absorption from skin, and the absorption characteristics of skin vary greatly between species. The safety of RECUVYRA in cats, horses, or other species has not been evaluated.
WARNINGS
Human Safety:
Not for use in humans. Keep out of reach of children. Avoid unprotected contact with application site for 72 hours.
SECONDARY EXPOSURE TO FENTANYL: Strict adherence to the requirements of the RiskMAP and the INSTRUCTIONS FOR USE provided in this product insert is imperative in order to reduce the potential of secondary exposure to fentanyl from RECUVYRA treated skin.
Adult Human User Safety while handling and applying RECUVYRA in the Hospital:
Two trained staff for administration:
Do not dispense RECUVYRA for administration at home by the pet owner. RECUVYRA should only be handled and administered to dogs by hospital staff specially trained in its use. To prevent human adverse reactions or abuse, at least 2 trained administrators should be present during administration of RECUVYRA.
Protective covering:
To prevent direct contact of RECUVYRA with human skin or mucous membranes when handling and/or applying the solution, wear impermeable latex or nitrile gloves, protective glasses and a laboratory coat. To avoid inadvertent contamination of other humans or animals, remove and appropriately dispose of protective garments after RECUVYRA minimum drying time of 5 minutes.
Mucous membrane or eye contact during administration: Direct contact of RECUVYRA with the eyes, oral cavity or mucous membranes of dogs or humans could result in systemic, clinically relevant fentanyl concentrations. If accidental eye, oral or other mucous membrane contact is made during administration, flush the area with water and seek immediate medical attention.
Skin contact during administration:
If human skin is accidentally exposed to RECUVYRA, wash the exposed area with soap and water and seek medical attention immediately. Accidental exposure could cause adverse reactions.
Drying time:
Following application to the dog, allow a minimum drying time of 5 minutes. As a precaution, hospital staff responsible for handling the dog prior to, during, and after surgery, should wear gloves. All others (including owners) should avoid contact with the application site for 72 hours following application. Within this period, any part of the body that directly contacts the application site should be washed with soap and water and not placed in the mouth.
User Safety following discharge of the dog to the owner:
Adult exposure:
Adults should avoid contact with the application site for 72 hours (3 days) following the application of RECUVYRA to the dog. Within this period, any part of the body that directly contacts the application site should be washed with soap and not placed in the mouth.
Child exposure:
The dog should be isolated from children for 72 hours (3 days) from the time of RECUVYRA application to the dog.
If a child comes in direct contact with the dog within 72 hours (3 days) from the time of RECUVYRA application to the dog, the exposed part of the child's body should not contact the child's mouth or eyes, and the area should be washed with soap and water.
If a child's tongue comes in contact with the dog, or another part of the child's body comes in contact with the dog and is then placed in the mouth, it is possible for fentanyl to enter the bloodstream; this is a medical emergency and the child should be seen immediately by a physician.
Drug abuse, addiction and diversion of opioids:
RECUVYRA contains fentanyl, a μ-opioid agonist and a Class II controlled substance with high potential for abuse similar to hydromorphone, methadone, morphine, oxycodone, and
oxymorphone. Fentanyl can be abused and may be subject to misuse, and criminal diversion. RECUVYRA should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the clinical setting and as required by law.
Animal Safety:
Individual dogs sensitive to the effects of fentanyl may develop pronounced sedation, decreased food and water intake and gastrointestinal (GI) stasis, which may increase the risk of bacterial overgrowth. Dehydration and elevations in hematocrit, albumin and fibrinogen may occur. Feces, if present, could be soft and contain blood. If severe GI stasis occurs, the dog should be reversed with naloxone (see PRECAUTIONS) and administered supportive intravenous fluids.
RECUVYRA has not been evaluated in dogs with respiratory disorders, cardiovascular disease, renal disease, or hepatic disease.
Opioid effects, including adverse reactions, may last for 7 days beyond administration in some dogs. To prevent potential overdose and severe adverse reactions, do not administer other opioids within 7 days of RECUVYRA administration.
During general anesthesia following administration of RECUVYRA, dogs should be continuously monitored and facilities should be available for the maintenance of a patent airway, artificial ventilation, IV fluids, and oxygen supplementation. Hypothermia may be severe and prolonged (greater than 24 hours) necessitating the use of an external heat source during surgery, throughout recovery, and after recovery from anesthesia.
PRECAUTIONS
The concomitant use of RECUVYRA with other CNS depressants (sedatives, hypnotics, general anesthetics, phenothiazines, and skeletal muscle relaxants) may cause respiratory depression, hypothermia, bradycardia, hypotension, and profound sedation. When such concomitant therapy is used, the doses of these agents should be reduced.
Fentanyl is a potent anesthetic sparing drug. To avoid anesthetic overdose, anesthetic agents should be administered to effect in dogs treated with RECUVYRA.
RECUVYRA may result in hypothermia, hypoventilation, hypotension, and bradycardia during anesthesia. Rectal temperature, pulse rate, respiratory rate, oxygen saturation, heart rhythm and blood pressure should be monitored during anesthesia.
Dogs may need longer post-surgical monitoring because of hypothermia or prolonged sedation due to RECUVYRA (see ADVERSE REACTIONS). RECUVYRA may cause prolonged physiological effects (sedation, respiratory depression, hypothermia, bradycardia, and hypotension) lasting longer than 24 hours. Clinical effects (diarrhea, vomiting, anorexia) could also worsen on the day after surgery. Some dogs may need longer hospitalization, monitoring and appropriate treatment.
Moderate or severe sedation can last up to 2 days after dosing. Dogs must not be discharged to owners until post-operative sedation is mild or absent and dogs are drinking water and eating on their own at an appropriate level for the condition that required surgery.
Emergency administration of naloxone at 0.04 mg/kg may be used to reverse serious adverse reactions associated with RECUVYRA. Administer the reversal agent slowly to avoid abrupt increases in blood pressure and catecholamine levels1. Reversal should occur rapidly (1-2 minutes); however, the duration of action of naloxone is variable (1-3 hours)2. The effects of RECUVYRA could last longer than the effects of the opioid reversal agent.
Fentanyl is a substrate for both the cytochrome P450 3A12 in dogs and the p-glycoprotein membrane efflux transporter3. Therefore, the concomitant use of RECUVYRA with drugs that inhibit these systems, such as ketoconazole or ivermectin, or the use of this drug in dogs with defective p-glycoprotein4, could result in higher than expected concentrations of fentanyl in the plasma and/or the brain, resulting in a
potential increase in adverse reactions (see CLINICAL PHARMACOLOGY).
RECUVYRA has not been evaluated in dogs younger than 6 months of age or in geriatric dogs.
RECUVYRA has not been evaluated for use in breeding, pregnant, or lactating dogs.
ADVERSE REACTIONS
In two randomized multi-centered, double-masked, active-controlled field studies across 25 investigative sites, 502 dogs had orthopedic or soft tissue surgery and were allotted to RECUVYRA (N=249) or oxymorphone hydrochloride (N=253). No RECUVYRA-treated dogs were removed from the study due to adverse reactions under anesthesia. The following adverse reactions were reported in RECUVYRA-treated dogs:
*Physiological adverse reactions occurred during general anesthesia and were included if there was at least one excursion outside the normal anesthetic range at any 5 minute interval during the entire duration of anesthesia. | ||
| Adverse Reaction* | RECUVYRA (N=249) | |
| Tachypnea (>20 breaths/min) | 158 |
(63%) |
| Bradypnea (<10 breaths/min) | 116 |
(47%) |
| Hypertension | 37 |
(15%) |
| Hypotension | 32 |
(13%) |
| Tachycardia (>180 beats/min) | 26 |
(10%) |
| Hypothermia (< 95°F) | 23 |
(9.2%) |
| Bradycardia (< 50 beats/min) | 9 |
(3.6%) |
| Pyrexia (>102.5°F) | 3 |
(1.2%) |
| Cardiac Arrhythmia | 2 |
(0.8%) |
| Reduced Oxygen Saturation of | 2 | (0.8%) |
| Hemoglobin (pulse oximetry < 85%) | ||
* Day 0 is the period following extubation on the day of the surgical procedure. | |||||
| Adverse Reactions: | Day 0* | Day 1 | Day 2 | Day 3 | Day 4 |
| RECUVYRA (N=249) | N (%) | N (%) | N (%) | N (%) | N (%) |
| Sedation | 123 (49%) | 8 (3.2%) | 1 (0.4%) | 0 (0%) | 0 (0%) |
| (moderate or severe) | |||||
| Diarrhea | 1 (0.4%) | 5 (2%) | 2 (0.8%) | 1 (0.4%) | 0 (0%) |
| Emesis | 0 (0%) | 4 (1.6%) | 2 (0.8%) | 2 (0.8%) | 0 (0%) |
| Hypothermia | 4 (1.6%) | 10 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pyrexia | 0 (0%) | 0 (0%) | 1 (0.4%) | 1 (0.4%) | 0 (0%) |
| Anorexia | 0 (0%) | 2 (0.8%) | 1 (0.4%) | 0 (0%) | 0 (0%) |
| Death | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.4%) | 0 (0%) |
RECUVYRA-treated dogs that were moderately or severely sedated on the day of surgery ranged between 49% at 1 hour post-extubation, 7% at 12 hours post-extubation, and 3% by 24 hours post-extubation. All dogs were mildly or not sedated from 60 hours through 96 hours following surgery.
One 13 year old dog in the fentanyl group was found dead three days after surgery. The dog had a history of vomiting for 4 days prior to the study. Necropsy revealed the cause of death was bacterial pneumonia from repeated vomition. The death was not attributed to fentanyl. One oxymorphone-treated dog died during the study and the death was the result of pulmonary embolism and stroke.
To report adverse drug reactions, for technical assistance, or to obtain a Material Safety Data Sheet (MSDS), contact Elanco Animal Health at 1-888-545-5973.
Adverse drug reactions may also be reported to the FDA/CVM at 1-888-FDA-VETS or http://www.fda.gov/AnimalVeterinary/SafetyHealth
INFORMATION FOR DOG OWNERS
Prior to administration with RECUVYRA, dog owners should understand the safety outcomes that could affect them, their family, and their dog. A client information sheet should be discussed and sent home with every dog treated with RECUVYRA. The RECUVYRA RiskMAP program considers the client information sheet as an informed consent form for the dog owner and contains a signature line which should be signed prior to use to document that the dog owner has read and understood the client information sheet.
The owner should be able to isolate the dog from family members, especially children, for several days after discharge. Contact or suspected contact in children could result in potential adverse reactions associated with systemic fentanyl blood levels and should be treated as a medical emergency.
Adverse reactions in the dog may include excessive sedation, decreased appetite, vomiting, and diarrhea. Serious adverse reactions, including death, can occur if prolonged lack of food and water intake and sedation are not addressed with supportive care. Owners should be advised to contact their veterinarian immediately if these signs occur after the dog returns to the home environment (see Client Information Sheet).
CLINICAL PHARMACOLOGY
Fentanyl exerts its analgesic action by binding to and activating μ (mu) opioid receptors that are predominantly found in the pain regulating areas of the brain and spinal cord. The analgesic effects of RECUVYRA are related to the resulting fentanyl blood concentration achieved following application. Once applied to the skin, solvent evaporation results in super-saturation of both a penetration enhancer and fentanyl. At the moment of drying (approximately 2 - 5 minutes following application), rapid dermal absorption and sequestration of fentanyl into the stratum corneum occurs. From the stratum corneum, fentanyl is absorbed into the bloodstream. Because RECUVYRA is not a device like a dermal patch, external thermal heat pads or warm water circulating blankets may be used during or following anesthesia.
In dogs, following intravenous and subcutaneous administration, fentanyl citrate is highly metabolized, with metabolites recovered in the urine and feces5. Fentanyl metabolism has been associated with the cytochrome P450 isoform CYP3A12 in dogs6. In mice lacking P-glycoprotein (P-gp), the maximum fentanyl analgesic effect was increased and prolonged compared to mice with functional P-gp7. P-gp is involved in the active efflux of fentanyl out of the central nervous system, preventing excessive accumulation of fentanyl in the brain. Therefore, dogs with compromised P-gp could potentially experience a greater magnitude and duration of analgesic activity with an increased risk of adverse reactions.
Unlike parenteral injections of fentanyl citrate in dogs where the terminal half-life of fentanyl is determined by the elimination rate, fentanyl from RECUVYRA exhibits absorption-dependent (flip-flop) pharmacokinetics. The absorption rate of fentanyl from skin is much slower than the elimination rate from blood and therefore the primary factor responsible for prolonged fentanyl blood concentrations is extended absorption. Some RECUVYRA can remain under the skin, slowly moving to the blood for up to 1 week.
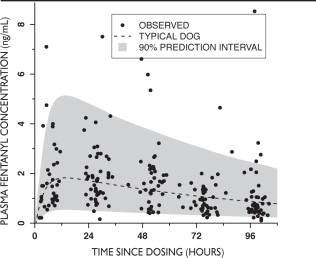
Pharmacokinetic parameters were calculated from 215 dogs undergoing orthopedic or soft tissue surgery. The mean plasma fentanyl concentration from time 0 through 96 hours post-dose administration was 1.32 ng/mL (Figure 1).
EFFECTIVENESS
In two randomized multi-centered, double-masked, active-controlled field studies across 25 investigative sites, dogs were randomized to RECUVYRA group (N = 249) or opioid active control group (oxymorphone hydrochloride; N = 253 dogs). Dogs in the RECUVYRA group received a single, topical application at a dose of 1.2 mg/lb (2.7 mg/kg) onto the dorsal scapular skin 2 – 4 hours prior to orthopedic or soft tissue surgery. Acepromazine was the most commonly used preanesthetic. Surgeries included cruciate ligament repair, ovariohysterectomy, lateral ear resection, laparotomy, liver biopsy, kidney removal, and tumor removal. Pain was quantified by a trained observer for 96 hours following surgery. At any time point, if the dog's pain was scored as inadequately controlled, the dogs were considered treatment failures and received supplemental analgesia. Any dogs given an emergency opioid reversal agent for adverse reactions were also considered treatment failures. A total of 21 dogs were treatment failures (4 in the RECUVYRA group and 17 in the active control group). No RECUVYRA-treated dogs were removed due to adverse reactions. The 4 RECUVYRA-treated treatment failures were withdrawn due to inadequate pain control between 1 and 6 hours post-extubation. A non-inferiority evaluation of the upper bound margin of difference between RECUVYRA and active control treatment failure rates over 96 hours demonstrated that RECUVYRA was non-inferior to the active control. Therefore RECUVYRA was effective in controlling pain associated with orthopedic and soft tissue surgery.
ANIMAL SAFETY
In a 16 week laboratory animal safety study, RECUVYRA was administered to young, healthy, adult mongrel dogs at 0 mg/kg (2 dogs), 2.6 mg/kg (8 dogs), 5.2 mg/kg (4 dogs) and 7.8 mg/kg (4 dogs). Doses correspond to 0, 1, 2, and 3 times the dose respectively, and were administered every 7 days for 16 weeks. All doses were applied to the dorsal scapular area. There were no deaths during the study. Sedation was observed with greater frequency and severity in the 5.2 and 7.8 mg/kg groups than in 2.6 mg/kg group. In the 2.6 mg/kg group, moderate sedation was noted in 2 dogs on day 1 and in a single dog on days 2, 14, and 56. Otherwise, sedation was mild or absent. Profound sedation was observed in a single dog in the 5.2 and 7.8 mg/kg groups on days 1-3. The increase in the mean sedation scores was greater during the first few dose intervals than in the subsequent dose intervals, showing the development of tolerance to the sedative effects of RECUVYRA. Evidence of tolerance to RECUVYRA's effects on heart rate (HR) or respiratory rate (RR) were not definitive; however, the largest decreases in HR and RR occurred after the first dose, generally lessening over time with subsequent doses.
Dogs received supportive therapy as needed during the study. Canned food, towels, or fluid therapy were provided to some dogs (including the 2.6 mg/kg dose group) to prevent weight loss, hypothermia, or moderate sedation. The day after dosing showed the greatest overall decreases in body temperature. By the second day after dosing, the lowest temperature noted in the 2.6 mg/kg group during the 16 week study was 99.1ºF. Dogs in the 2.6 group did not receive any temperature related supportive therapy; some dogs in the 5.2 and 7.8 mg/kg groups received thermal support during the study (hot water bottles, heated pads). Dogs had bradycardia (<70 bpm) and moderate sedation on the day of dosing and/or the first day after dosing. In treated groups (including 2.6 mg/kg dogs), HR were lower on the day after dosing compared to 12 hours after the administration of fentanyl. HR decreases did not require treatment and were not severe in the 2.6 mg/kg group. Decreases in RR was seen in all treated groups but bradypnea (RR <10) was not seen. The lowest RR in the 2.6 and 7.8 mg/kg groups was 12 breaths per minute (bpm); the lowest RR in the 5.2 mg/kg group was 16 bpm.
Diarrhea was more frequently observed in fentanyl-treated dogs; however, control dogs also had diarrhea. Six dogs had blood in the stool (described as hemorrhagic diarrhea, tarry stool), including 2 dogs in the 2.6 mg/kg dose group. Four of the 6 dogs with blood in the stool had mild elevations in WBC (13 dogs overall had increases in WBC). Four dogs had elevations during week 1 (0, 2.6, and 5.2 mg/kg), and 2 dogs during week 2 (0 and 7.8 mg/kg).
Inappetance (not due to excessive sedation) and weight loss were seen frequently in the treated dose groups. Body weights dropped initially, through the first to second weeks, returning toward baseline by week 5. In the 2.6 mg/kg group (8 dogs), weight loss during the first two weeks ranged from 5.2-15.8% of initial body weight (compared to 1.7-6.5% in the 2 control dogs). Five of 8 lost 5-<10%; 2 dogs lost 10-<15%; and 1 dog lost 15.8% of body weight compared to baseline body weights. Abnormalities associated with increased WBC, decreased HGB (hemoglobin), and decreases in MCHC (mean corpuscular hemoglobin concentration) were mild, occurring in the control dogs and all dose groups including the 2.6 mg/kg group. Gross necropsy findings for all treatment groups did not reveal any treatment related findings and no histopathologic findings were reported in the 2.6 mg/kg group. Fentanyl-related hepatic changes consisted of brown pigmentation in 1 dog from the 5.2 mg/kg and 2 dogs from the 7.8 mg/kg group, focal granulomas in 1 dog from the 7.8 mg/kg group, and single cell necrosis identified in 1 dog from the 7.8 mg/kg group; all changes were considered reversible.
In another safety study, RECUVYRA was administered to 32 healthy adult mongrel dogs (8 dogs per group) at 0 mg/kg, 2.6 mg/kg, 5.2 mg/kg, and 7.8 mg/kg as a topical application to the ventral abdomen every 4 days for a total of 3 doses. Application to the ventral abdomen results in approximately 30% greater exposure with similar peak exposure, compared to dorsal scapular application.
Three dogs died as a result of RECUVYRA administration. Two dogs died on days 4 and 7 (5.2 mg/kg group), and 1 dog died on day 5 (7.8 mg/kg group), all between the second and third doses. Death was due to endotoxic shock as a result of severe gastrointestinal stasis complicated by continued hypothermia, severe dehydration, prolonged sedation, low HR and RR, and elevated clotting parameters.
Sedation varied from slight to profound within all 3 treated groups. Sedation scores were highest during the second dose period and occurred more quickly after the second and third doses. In the 2.6 mg/kg group, 3 dogs showed no or slight sedation, 5 dogs showed moderate, with 2 (of these 5) showing profound sedation. Most dogs in the 5.2 mg/kg and 7.8 mg/kg groups remained moderately to profoundly sedated.
Heart rates and respiratory rates began decreasing within 4 hours of dosing. Respiratory rates had a downward trend with each subsequent dose. Lowest heart rates in the 2.6, 5.2, and 7.8 mg/kg groups were 28, 16, and 28 beats per minute, and the lowest respiratory rates were 8, 4, and 4 breaths per minute. ECG findings in treated dogs included bradycardia and increases in PR and QT intervals. One dog in the 5.2 mg/kg group showed secondary atrioventricular (AV) block.
In the 2.6 mg/kg group, dogs became hypothermic (body temperature <98°F) within 12 hours after the first dose. After the second and third doses, some dogs (in all treatment groups) developed hypothermia by 4 hours. The overall largest decrease in mean body temperatures occurred 12-24 hours after dosing. Hypothermia lasted 1-3 days in the 2.6 mg/kg group, and up to 4 days in the 5.2 and 7.8 mg/kg groups. Temperatures as low as 89, 84.2, and 92.1°F in the 2.6, 5.2, and 7.8 mg/kg groups, respectively, were recorded.
Other adverse reactions in all treated groups were salivation, vomiting, and mydriasis. Fecal abnormalities included blood in the feces, diarrhea, and lack of feces. Blood in the feces increased in severity with increased dose and repeated doses. In the 2.6 mg/kg group, 2 dogs had blood in the feces once after the first or second dose; a third 2.6 mg/kg dog had 4 observations after the second dose and 10 observations after the third dose.
Daily food consumption and body weight decreased after fentanyl dosing, especially in the higher dose groups. In the 2.6 mg/kg group, food consumption decreased 87% after the first dose, then 70% and 37% after doses 2 and 3, returning to pre-treatment food consumption by day 12. Dogs lost the most body weight in the first dose period. In the 2.6 mg/kg group, three dogs lost 19-20% and three dogs lost 5-10% body weight. All dogs in the 5.2 and 7.8 mg/kg groups lost between 10-21% body weight.
Increases in mean hematocrit, hemoglobin, red blood cell count, albumin, globulin, and total protein values were consistent with dehydration and most pronounced in the 2 higher dose groups. These values were highest on day 4, dropping below day 0 mean values by the end of the study. Three of 8 dogs in the 2.6 mg/kg group had reticulocyte decreases of approximately 85% at day 12 compared to day 0 with markedly lower values in the 5.2 and 7.8 mg/kg groups by days 8 and 12. Other clinically relevant clinical pathology changes included above normal WBC counts (3 dogs in the 7.8 mg/kg group), above normal APTT values in 2 dogs in each of the higher dose groups, and dose related increases in fibrinogen values in all 3 groups, peaking at day 4 and decreasing thereafter.
Necropsy findings included multifocal intestinal ulceration (moderate necrosis) in 1 dog (5.2 mg/kg), multifocal intestinal erosions in 1 dog (5.2 mg/kg), and mucosal congestion (with red or black intestinal contents) in 2 dogs. All 3 dogs that died had diffuse proteinaceous casts in the renal tubules. Bone marrow evaluation (not performed in the 3 dogs that died) showed changes consistent with an increased demand for white blood cells, including dogs in the 2.6 mg/kg group.
STORAGE INFORMATION
Store at controlled room temperature, 20 - 25ºC (68 - 77ºF). Once broached with the needleless adaptor, store the vial upright at controlled room temperature, 20 - 25ºC (68 - 77ºF), with the needleless adaptor attached. Discard broached vials after 30 days.
REFERENCES
1Mills CA, et al. J Anesth. Narcotic reversal in hypercapnic dogs: comparison of naloxone and nalbuphine. 1990, 37(2): 238-244.
2 Plumb DC. In: Plumb's Veterinary Drug Handbook, 5th edition. Ames: Blackwell Publishing, 2005;547.
3 Mealey KL, Therapeutic implications of the MDR-1 gene. J Vet Pharmacol Therap. 2004, 27:257-264.
4 Mealey KL, Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics 2001, 11:727-733.
5 Otsuka H, et al. Pharmacokinetics of fentanyl after intravenous and subcutaneous administration to rats and dogs. Jpn Pharmacol Ther. 2001, 29(11):877-886.
6 Lu PL, et al. Selective Inhibition of Dog Hepatic CYP2B11 and CYP3A12 J Pharmacol Exp Ther. 2005, 313(2):518-528.
7 Thompson SJ, et al. Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000 May; 92(5):1392-1399.
HOW SUPPLIED
RECUVYRA is supplied in 10 mL amber-colored glass vials
(50 mg fentanyl/mL). Each vial is supplied with a purpose-designed needleless adaptor, syringes and applicator tips.
NADA 141-337, Approved by FDA NDC 0986-4232-11
Manufactured for Elanco Animal Health,
A Division of Eli Lilly and Company
Indianapolis, IN 46285
CA423210HAM
PA087463AMX (Aug 2013)
Elanco and Recuvyra are trademarks owned or licensed by Eli Lilly and Company, its subsidiaries or affiliates.
ElancoTM
CLIENT INFORMATION SHEET and CONSENT FORM For:
Recuvyra™
(fentanyl)
transdermal
solution
CII
For Topical Application in Dogs Only
Opioid Analgesic
NOT FOR INJECTION
50 mg/mL (500 mg/10 mL )
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
RECUVYRA (pronounced “RI-KUHV′-I-RA”)
is indicated for the control of postoperative pain associated with surgical procedures in dogs
Established name: fentanyl (fen′tah-nil)
This summary contains important information about RECUVYRA. You should read this information if your veterinarian gives RECUVYRA to your dog to control the pain after surgery. This summary helps you remember your veterinarian's instructions as they relate to your safety, the safety of your family members, and the care of your dog after surgery. Ask your veterinarian to explain this information or to give you more information about RECUVYRA.
|
WARNING: Abuse Potential: RECUVYRA contains fentanyl, which is a potent narcotic. Fentanyl is a controlled substance and has a high possibility for abuse. Risk Minimization and Action Plan: This product is distributed under a Risk Minimization Action Plan (RiskMAP) and its use is limited to certified veterinarians. Human Safety: SECONDARY EXPOSURE OF FENTANYL IN CHILDREN AND ADULTS: To prevent the transfer of fentanyl from the RECUVYRA application site on your dog to children, you must strictly follow these warnings:
Animal Safety: The most common side effects seen following surgery in dogs were: frequent or constant sleepiness, diarrhea, vomiting, low body temperature, abnormal heart rhythms, fever and lack of appetite. Some dogs that are especially sensitive to the effects of fentanyl may develop a slowdown or stoppage of bowel movement. Bowel movements, if present, could be soft and contain blood. If severe side effects happen, contact your veterinarian immediately. Your veterinarian can treat your dog with drugs to reverse the effects of fentanyl. |
What is a Risk Minimization Action Plan ( RiskMAP)?
The use of RECUVYRA has known risks in humans and dogs. To minimize the known risks while preserving benefits, a RiskMAP is being instituted. A RiskMAP targets safety related goals and uses tools to achieve those goals. One of the tools of the RECUVYRA RiskMAP is to provide specific training to veterinarians and staff that handle RECUVYRA. Before purchase, clinic staff that will handle or apply RECUVYRA must have documented training and certification in its use.
This information sheet is considered an informed consent document and is part of the RiskMAP. You must read and understand this information prior to the use of RECUVYRA on your dog. Make sure your veterinarian answers all of your questions prior to signing this document.
Can I pet my dog over the shoulder blade area where my veterinarian administered RECUVYRA?
Adults should avoid contact with the application site for 72 hours (3 days) following the application of RECUVYRA to your dog. Within this period, if any part of your body directly contacts the application site, the exposed skin should be washed with soap and water and not inserted into your mouth. Other than the area where RECUVYRA was applied to your dog, you can touch your dog as normal.
What is RECUVYRA?
RECUVYRA is a prescription pain-relieving drug for dogs. This drug is not for use in humans and not for use in any animal species except for dogs. The active ingredient, fentanyl, is a potent narcotic, called an opioid. Because it is a controlled substance (like morphine), it cannot be given by anyone except your veterinarian in the animal hospital.
| RECUVYRA application | ||
| Date | Time | Amount |
| Applied | Applied | (milliliters) |
Children should not have contact with the dog until:
Can children pet the dog when it returns home?
IT IS IMPORTANT THAT YOU KEEP CHILDREN AWAY FROM
THE DOG FOR 72 HOURS FROM THE TIME OF APPLICATION
Children should not be allowed to contact the dog for a minimum of 72 hours (3 days) after RECUVYRA is applied to the dog. If a child touches the dog, do not let the exposed area (for example, fingers) contact the child's mouth or eyes, and wash the area with soap and water.
If a child's tongue comes in contact with the dog, or another part of the child's body comes in contact with the dog and is then placed in the mouth, it is possible for RECUVYRA to enter the child's bloodstream and cause serious side effects, including breathing problems and sleepiness. This is a MEDICAL EMERGENCY and the child should be seen immediately by a physician. Give this information sheet to the child's physician.
In case of human exposure emergency, call 1-800-722-0987. What are the possible side effects that could happen after my dog is given RECUVYRA?
RECUVYRA may cause side effects. The most common side effects are lack of appetite and decreased activity due to sleepiness. Your dog might also lose weight, vomit, have diarrhea, or feel cold to the touch (for example, the ears). These effects are most noticeable during the first 24 to 48 hours following surgery but may continue for several days. Some dogs may need longer post-surgical monitoring because of reduced body temperature or prolonged sleepiness. As a result, some dogs may require longer hospitalization. Your veterinarian may recommend longer hospitalization until your dog is no longer excessively sleepy and is drinking water and eating, depending on the type of surgery that was performed on your dog.
Call or return to your veterinarian if:
- you cannot wake your dog or the dog sleeps all the time
- your dog will not eat or drink at all
- you see blood or reddish brown liquid in your dog's feces
- your dog has watery feces How is RECUVYRA given?
Two to four hours before your dog has surgery, a small amount of RECUVYRA is placed directly on the skin between the dog's shoulder blades. Within 5 minutes, fentanyl is absorbed under the skin and the skin dries. Fentanyl gradually moves from beneath the skin into your dog's bloodstream. Once in the blood, it relieves pain. A single dose relieves pain for 4 days. However, some RECUVYRA can remain under the skin, slowly moving to the blood for up to 1 week.
How might my dog behave at home after treatment with RECUVYRA?
RECUVYRA is a strong pain reliever. Depending on the type of surgery that your dog had, your dog may appear:
- Content, comfortable or quiet
- Moving about normally
- Not bothered by the area that had surgery
- Relaxed when you approach or interact with the surgical area
If my dog is still painful 4 days beyond the time of application of RECUVYRA, can my veterinarian administer another dose?
For any single surgery, RECUVYRA should be administered one time only. Repeated administrations could result in severe adverse reactions, including death.
Can RECUVYRA be given with other medicines?
Talk to your veterinarian about all medicine that you want to give your dog during the week after surgery (even those that do not need a prescription). Do not place any medication, including flea and tick medicine, on the shoulder blade area without first talking to your veterinarian. Your veterinarian should check your dog's other medicines to see if there is any possibility of an interaction.
What should I discuss with my veterinarian before my dog receives RECUVYRA?
To decide if RECUVYRA is the best pain reliever for your dog, your veterinarian needs information from you.
Tell your veterinarian:
- the dog's age
- if the dog is pregnant or has puppies
- if the dog is sick or was sick in the past
- if the dog has ever had breathing, heart, or blood pressure problems
- if the dog has ever had bowel or kidney problems
- what medicine the dog takes or has taken, especially within the last month
What else should I know about RECUVYRA?
If you have other questions or concerns about RECUVYRA or postoperative pain, talk to your veterinarian. Your veterinarian will best determine if your dog is responding as expected.
To report an adverse drug event (side effect), talk to your veterinarian or call Elanco Animal Health at 1-888-545-5973.
Adverse drug events may also be reported to the FDA/CVM at 1-888-FDA-VETS or http://www.fda.gov/AnimalVeterinary/SafetyHealth
I,__________________________,
( Print name of Owner or designated representative )
have read and understood the Client Information Sheet, and all my questions have been answered to my satisfaction by my veterinarian. I authorize my veterinarian to administer RECUVYRA to my dog,
__________________________
( Name of dog or ID number )
Signed: __________________________
( Owner or designated representative ) Date:
Date: __________________________
Attending Veterinarian: __________________________
Date: __________________________
Issues MONTH YEAR:: __________________________
CA4232
PA087169AMX
(April 2013)
Elanco and Recuvyra are trademarks owned or licensed by Eli Lilly and Company, its subsidiaries or affiliates.TM
Elanco™
Principal Display Panel - Kit Label
RecuvyraTM
(fentanyl) transdermal solution CII
For Topical Application in Dogs Only Opioid Analgesic
ACCESSORY KIT:
5 SYRINGES
5 APPLICATOR TIPS
ElancoTM
Principal Display Panel - Carton Label
RecuvyraTM
(fentanyl) transdermal solution CII
For Topical Application in Dogs Only Opioid Analgesic
NOT FOR INJECTION
WARNING: Due to serious human and animal safety concerns, Recuvyra (fentanyl) is distributed
under a Risk Minimization Action Plan and its use is limited to certified veterinarians.
Read the entire product insert before using this drug including the complete Boxed Warning.
50 mg/mL (500 mg/10 mL)
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Principal Display Panel - Vial Label
RecuvyraTM
(fentanyl) transdermal solution CII
For Topical Application in Dogs Only
Opioid Analgesic
NOT FOR INJECTION
WARNING: Due to serious human and animal safety
concerns, Recuvyra™ (fentanyl) is distributed under a
Risk Minimization Action Plan and its use is limited to
certified veterinarians. Read the entire product insert before
using this drug including the complete Boxed Warning.
50 mg/mL (500 mg/10 mL)
CAUTION: Federal law restricts this drug to use by or
on the order of a licensed veterinarian.